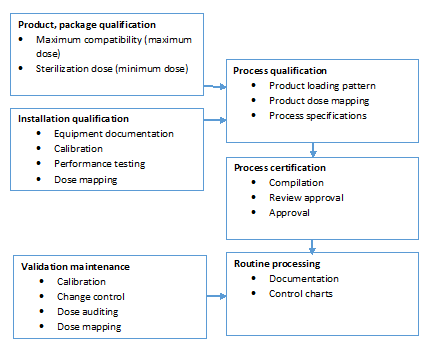
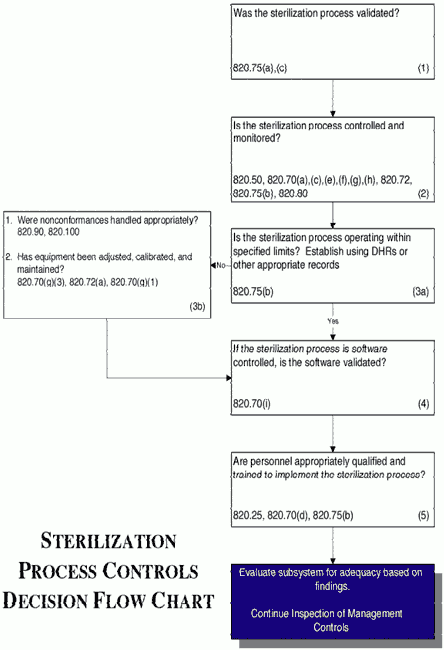
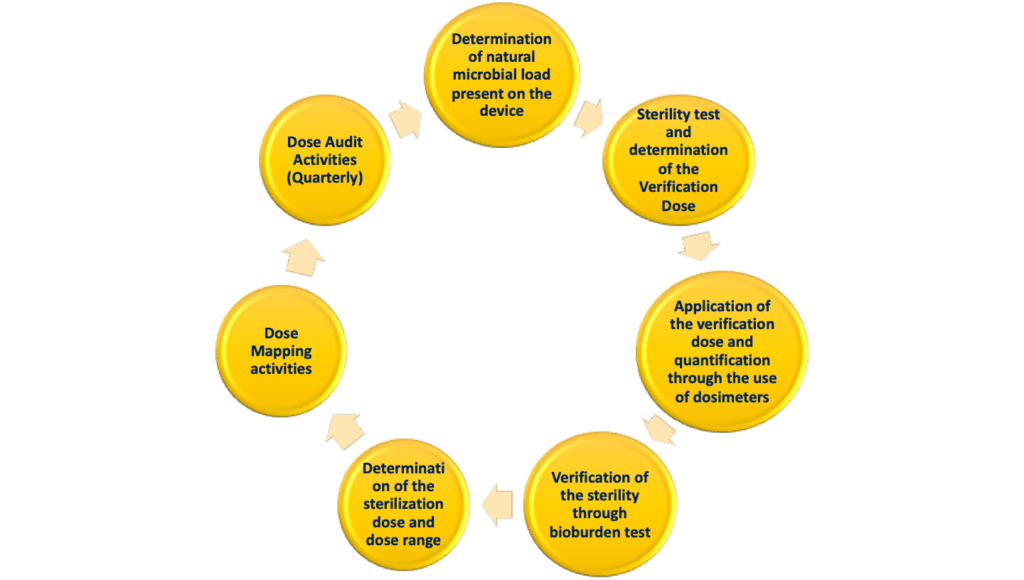
Gamma Irradiation: Sterilization Validation Approach for Pall Biopharmaceutical Filters with Sterile Claim

STERIS Applied Sterilization Technologies - Through locations in Europe, the United Kingdom and United States, our Radiation Technology Centers are dedicated to meeting the validation, dose audit, and research needs of our

Guide to Irradiation and Sterilization Validation of Single-Use Bioprocess Systems - BioProcess InternationalBioProcess International