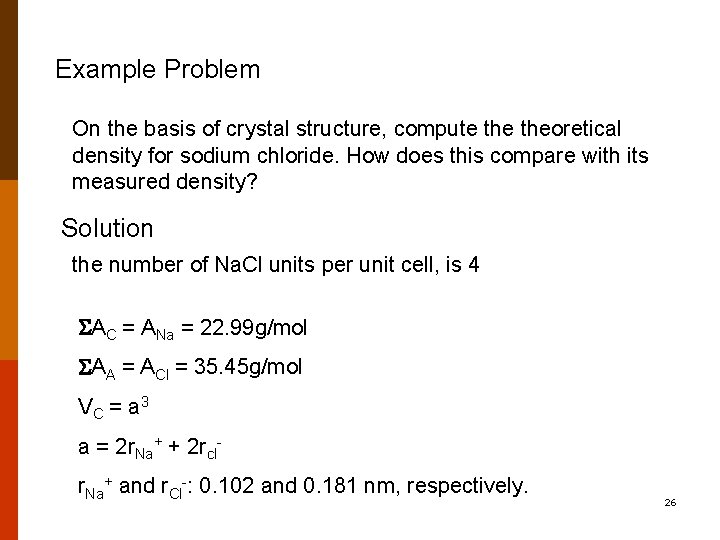
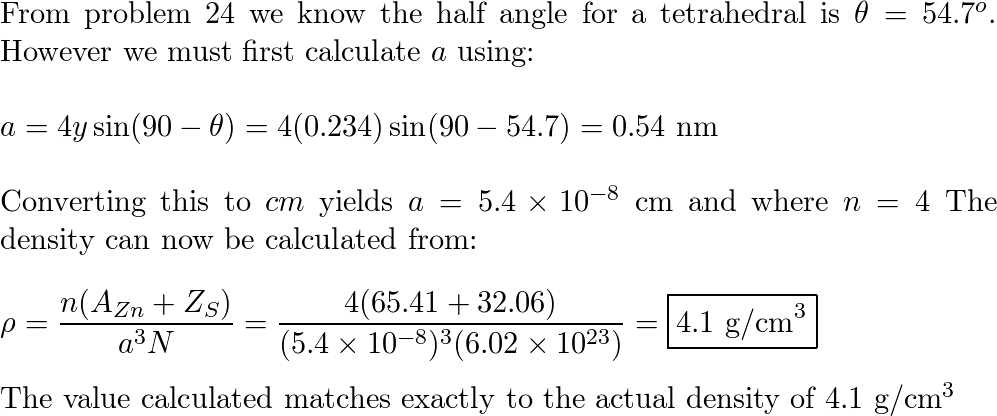SOLVED:Compute the theoretical density of ZnS, given that the Zn—S distance and bond angle are 0.234 nm and109.5, respectively. How does this value compare with the measured density?

Module 6.docx - Module 6 1. The number of vacancies in some hypothetical metal increases by a factor of 6 when the temperature is increased from 958 C | Course Hero

Module 6.docx - Module 6 1. The number of vacancies in some hypothetical metal increases by a factor of 6 when the temperature is increased from 958 C | Course Hero
![The density of ZnS crystal (Zinc blende structure) having 10% Frenkel defect is: [ rZn^2 + = 40√(3) pm,rS^2 - = 110√(3) pm ] (Z = 65.2, S = 32) The density of ZnS crystal (Zinc blende structure) having 10% Frenkel defect is: [ rZn^2 + = 40√(3) pm,rS^2 - = 110√(3) pm ] (Z = 65.2, S = 32)](https://haygot.s3.amazonaws.com/questions/1156438_993819_ans_74ad3ebcaf3b4a399711de2b301ae663.jpeg)
The density of ZnS crystal (Zinc blende structure) having 10% Frenkel defect is: [ rZn^2 + = 40√(3) pm,rS^2 - = 110√(3) pm ] (Z = 65.2, S = 32)

From Wurtzite Nanoplatelets to Zinc Blende Nanorods: Simultaneous Control of Shape and Phase in Ultrathin ZnS Nanocrystals | The Journal of Physical Chemistry Letters

Composition dependence in mechanical properties of zinc-blende compounds associated with the CdxZn1–xSyTe1–y system: a density functional study | SpringerLink

Compute the theoretical density of ZnS given that the Zn-S distance and bond angle are 0.234 nm and - Brainly.com

Density functional theory in the solid state | Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences
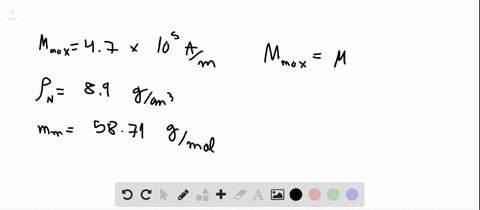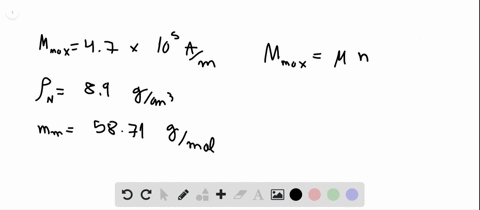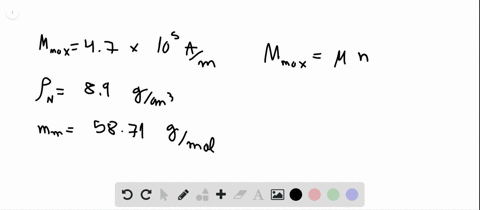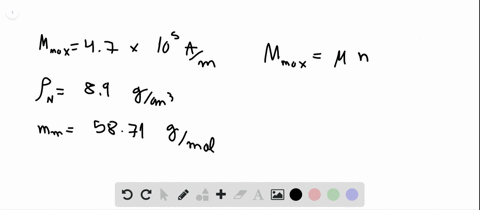
SOLVED:Compute the theoretical density of ZnS given that the \mathrm{Zn}-\mathrm{S} distance and bond angle are 0.234 \mathrm{nm} and 109.5^{\circ}, respectively How does this value compare with the measured density?

Module 6.docx - Module 6 1. The number of vacancies in some hypothetical metal increases by a factor of 6 when the temperature is increased from 958 C | Course Hero

Theoretical investigation of magnesium and selenium concentration dependent elastic properties of zinc blende specimens under the MgxZn1-xSeyTe1-y quaternary system with density functional FP-LAPW approach - ScienceDirect